
Analyzing the April 1st, 2022, ICD-10-CM/PCS Updates
The Centers for Medicare and Medicaid Services (CMS) announced in the Hospital Inpatient Prospective Payment System (IPPS) final rule, published on August 2, 2021, the adoption of a second implementation date of April first for code updates. This date was previously used for implementing diagnosis and/or procedure codes needed to describe new technologies and medical services for potential add-on payment. The updates for this second implementation date will be announced in November of the prior year and as promised, CMS released the new CM and PCS codes effective with discharges beginning April 1, 2022.
ICD-10-CM Code Updates Boost COVID-19 Immunization Tracking
The ICD-10-CM updates regard capturing COVID-19 vaccination status. Underimmunization for COVID-19 is a significant risk factor in contracting the illness. As you can imagine, the ability to collect data during a pandemic is critical in preparing for future health crises. Tracking people seeking healthcare who are unimmunized or partially immunized will help with clinical research, not to mention enhance public health safety. A new subcategory Z28.31- titled “Underimmunization for COVID-19 status” was developed to capture this data. The table below demonstrates the new ICD-10-CM tabular section. (CMS, 2021)
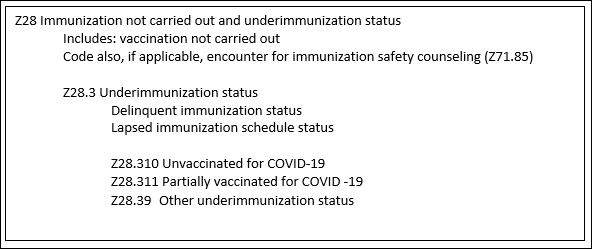
These three new codes will not impact the MS-DRG calculation as they are designated non-comorbid conditions (non-CC).
Related: Webinar Recap: Introducing ICD-11 MMS
ICD-10-PCS Code Updates Cover COVID-19 Booster
The ICD-10-PCS code updates regard COVID-19 as well. Currently, there are procedure codes for the first and second doses of the COVID-19 vaccine. As of April first, there will be codes for a third dose as well as an option for a booster. These new codes appear in the New Technology, Anatomical Region, Introduction (XW0) PCS table and are listed below.
XW013V7 Introduction of COVID-19 vaccine dose 3 into subcutaneous tissue, percutaneous approach, new technology group 7
XW013W7 Introduction of COVID-19 vaccine booster into subcutaneous tissue, percutaneous approach, new technology group 7
XW023V7 Introduction of COVID-19 vaccine dose 3 into muscle, percutaneous approach, new technology group 7
XW023W7 Introduction of COVID-19 vaccine booster into muscle, percutaneous approach, new technology group 7
The graphic below identifies these codes in table form.
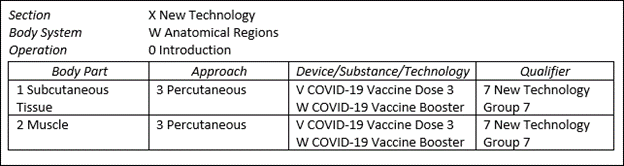
Since these new vaccine codes are designated non-O.R., they will not have an impact on the MS-DRG calculation. Medicare reminds us that they will pay for COVID-19 vaccinations administered to beneficiaries in an inpatient setting using CPT codes. As of January 1, 2022, specific manufacturer vaccine CPT codes went into effect. Facilities should make sure their chargemasters are updated with the codes listed below.
|
0001A Administration Pfizer first dose |
0022A Administration Janssen single dose |
|
0011A Administration Moderna first dose |
0041A Administration Noravax first dose |
|
0021A Administration of AstraZeneca first dose |
For additional assistance, refer to the American Medical Association CPT® 2022 professional edition code book appendix Q. This appendix provides links to the individual vaccine product code and their associated administration code, manufacturer name, vaccine name, national drug code, and dosage intervals. (AMA, 2022)

Fostamatinib Administered as a Treatment for COVID-19 Symptoms
The last of the April first updates involves the administration of fostamatinib to the arsenal of medications, such as remdesivir (antiviral) and dexamethasone (broad acting steroid) to treat COVID-19 symptoms. Marketed as Tavalisse®, fostamatinib is an oral spleen tyrosine kinase (SYK) inhibitor and has shown to inhibit a unique type of cell death associated with mortality in COVID-19 patients. SYK inhibits neutrophils and platelets, making it effective in decreasing thromboinflammation and potential organ dysfunction in hospitalized COVID-19 patients. Fostamatinib is administered orally or, if that isn’t possible, through an enteral feeding tube. (Hue, 2021) More on the science of this medication can be found in the ICD-10 Coordination and Maintenance Committee September 14, 2021, meeting minutes located on the Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) website.
These new PCS codes are listed below.
XW0DXR7 Introduction of fostamatinib into mouth and pharynx, external approach, new technology group 7
XW0G7R7 Introduction of fostamatinib into upper GI, via natural or artificial opening, new technology group 7
XW0H7R7 Introduction of fostamatinib into lower GI, via natural or artificial opening, new technology group 7
The graphic below identifies these codes in table form.
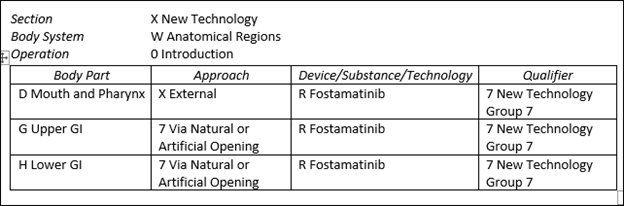
The above codes are also designated non-O.R. and will not impact MS-DRG assignment.
In Conclusion, the April first release cycle will have a phased-in approach with limited code updates and CMS does not feel there will be a need for new code books. In this article, I have only mentioned code updates, but coding guidelines may also be updated. The November notification date will give us plenty of time to educate coding professionals, clinical documentation improvement professionals, and physicians, ensuring a smooth transition.
References
- Centers for Medicare and Medicaid Services, (2021, November 16). DRG classifications and software. CMS.gov. Retrieved January 12, 2022, Download “ICD-10-DRGs V39.1 Effective April 1, 2022 (ZIP)”, “Web announcement_ ICD-10_MS-DRG_V39.1 New Diagnosis codes and )”, “Web announcement_ ICD-10_MS-DRG_V39.1 New procedure codes from https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software
- Hue, M., Andrea Hazeley, (2021, September 14). ICD-10 coordination and Maintenance Committee ... - cms.gov. Retrieved January 12, 2022, from https://www.cms.gov/files/document/september-2021-final-agenda-and-materials.pdf
- American Medical Association. (2022). AMA CPT® Professional 2022.
Latest News
Debunking Common Myths About CRNAs
Certified Registered Nurse Anesthetists (CRNAs) play an indispensable role in healthcare. They provide safe and effective anesthesia care to over 50 million patients per year in the United States,
Physician Assistant Specialties in Demand: Top Fields to Consider
If you're considering specializing or transitioning within your PA career, knowing which fields are in the highest demand can help propel your professional growth.
Nurse Practitioner Trends to Watch in 2025
The role of the nurse practitioner (NP) has never been more vital or celebrated. With a growing focus on value-based care, the increasing demand for healthcare providers, and the continuous
States with the Highest Paying CRNA Jobs in 2025
If you're a CRNA (or an aspiring one) seeking top-dollar opportunities, knowing which states offer the highest salaries can help focus your job search—whether you're settling down or
A Psychiatric NP’s Guide to Managing Patients with Complex Psychiatric Conditions
Psychiatric Nurse Practitioners (NPs) are at the forefront of mental health care, providing expert care to patients managing a wide range of psychiatric disorders. With the growing demand for
How PAs Can Take the Lead in Patient Advocacy
Physician assistants (PAs) wear many hats—clinician, teacher, collaborator, and more. But one of the most impactful roles they play, often overlooked, is that of a patient advocate. Advocacy
AI in the Operating Room: Opportunities and Challenges for CRNAs
The integration of artificial intelligence (AI) in healthcare has been revolutionary, and its impact is notably significant in the operating room. Certified Registered Nurse Anesthetists (CRNAs)
How Much Do Physician Assistants Make?
For 2024, the average physician assistant salary across the United States is continuing its upward trend. According to recent data, the average physician assistant salary is around $120,000. This











